Concepts, outils et méthodes pour la physico-chimie de formulation
Delforce, L.; Duprat, F.; Ploix, J.-L.; Ontiveros, J. F.; Goussard, V.; Nardello-Rataj, V.; Aubry, J.-M.
ACS Omega 2022, 7 (43), 38869–38881.
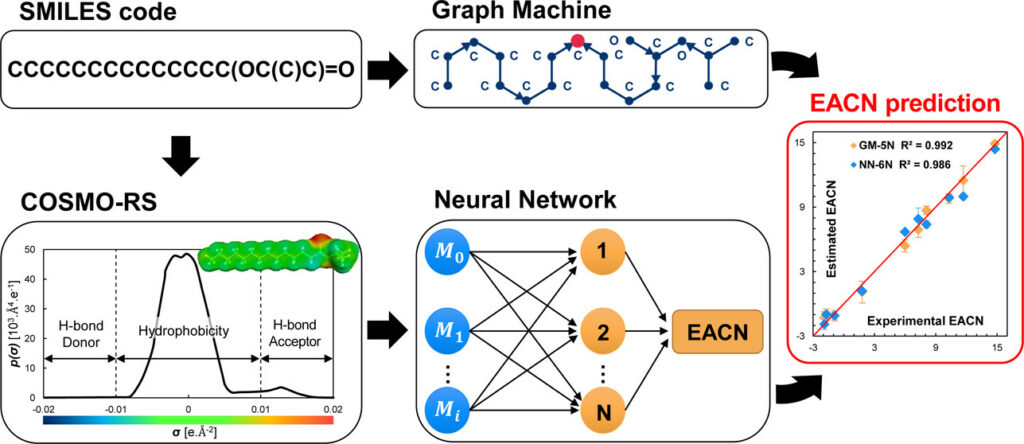 The hydrophobicity of oils is a key parameter to design surfactant/oil/water (SOW) macro-, micro-, or nano-dispersed systems with the desired features. This essential physicochemical characteristic is quantitatively expressed by the equivalent alkane carbon number (EACN) whose experimental determination is tedious since it requires knowledge of the phase behavior of the SOW systems at different temperatures and for different surfactant concentrations. In this work, two mathematical models are proposed for the rapid prediction of the EACN of oils. They have been designed using artificial intelligence (machine-learning) methods, namely, neural networks (NN) and graph machines (GM). While the GM model is implemented from the SMILES codes of a 111-molecule training set of known EACN values, the NN model is fed with some σ-moment descriptors computed with the COSMOtherm software for the 111-molecule set. In a preliminary step, the leave-one-out algorithm is used to select, given the available data, the appropriate complexity of the two models. A comparison of the EACNs of liquids of a fresh set of 10 complex cosmetic and perfumery molecules shows that the two approaches provide comparable results in terms of accuracy and reliability. Finally, the NN and GM models are applied to nine series of homologous compounds, for which the GM model results are in better agreement with the experimental EACN trends than the NN model predictions. The results obtained by the GMs and by the NN based on σ-moments can be duplicated with the demonstration tool available for download as detailed in the Supporting Information.
The hydrophobicity of oils is a key parameter to design surfactant/oil/water (SOW) macro-, micro-, or nano-dispersed systems with the desired features. This essential physicochemical characteristic is quantitatively expressed by the equivalent alkane carbon number (EACN) whose experimental determination is tedious since it requires knowledge of the phase behavior of the SOW systems at different temperatures and for different surfactant concentrations. In this work, two mathematical models are proposed for the rapid prediction of the EACN of oils. They have been designed using artificial intelligence (machine-learning) methods, namely, neural networks (NN) and graph machines (GM). While the GM model is implemented from the SMILES codes of a 111-molecule training set of known EACN values, the NN model is fed with some σ-moment descriptors computed with the COSMOtherm software for the 111-molecule set. In a preliminary step, the leave-one-out algorithm is used to select, given the available data, the appropriate complexity of the two models. A comparison of the EACNs of liquids of a fresh set of 10 complex cosmetic and perfumery molecules shows that the two approaches provide comparable results in terms of accuracy and reliability. Finally, the NN and GM models are applied to nine series of homologous compounds, for which the GM model results are in better agreement with the experimental EACN trends than the NN model predictions. The results obtained by the GMs and by the NN based on σ-moments can be duplicated with the demonstration tool available for download as detailed in the Supporting Information.
Lemahieu, G.; Ontiveros, J. F.; Gaudin, T.; Molinier, V.; Aubry, J.-M.
J. Colloid Interface Sci. 2022, 608, 549–563.
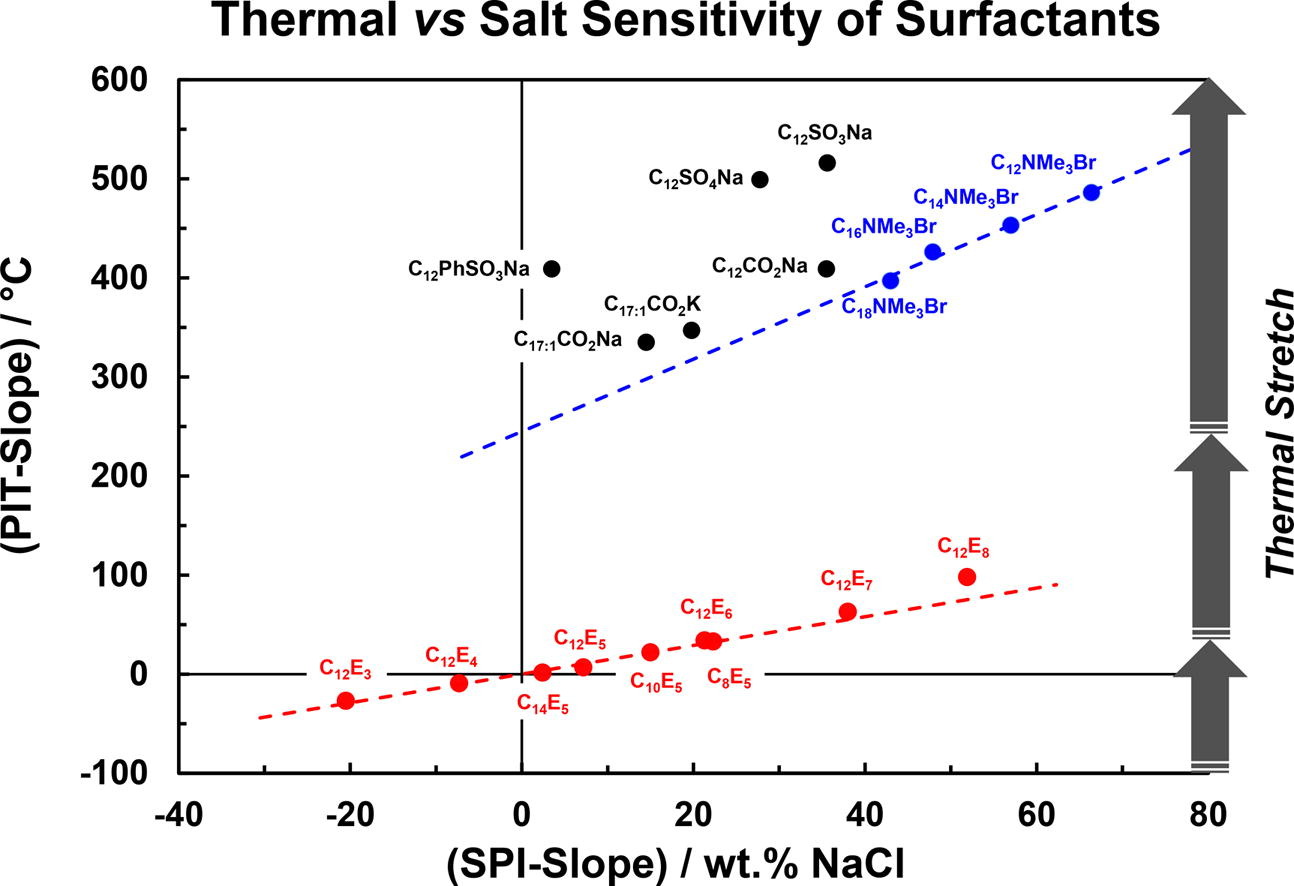
The salinity at which the dynamic phase inversion of the reference system C10E4/n-Octane/Water occurs in the presence of increasing amounts of a test surfactant S2 provides quantitative information on the hydrophilic/lipophilic ratio and on the sensitivity to NaClaq of S2.
The Salinities causing the Phase Inversion (SPI) of the reference system mixed with 12 ionic and 10 nonionic well-defined surfactants are determined in order to quantify the contributions of the nature of the polar head and of the alkyl chain length.
The SPI varies linearly upon the addition of S2. The slope of the straight variation with the molar fraction of S2 is called the “SPI-slope”. It quantifies the hydrophilic/lipophilic ratio of S2 in saline environment and its salt-sensitivity with respect to the reference surfactant C10E4. The SPI-slopes of C12 surfactants bearing different polar heads are found to decrease in the following order: C12NMe3Br > C12E8 > C12E7 ≥C12SO3Na ≈ C12COONa ≥ C12SO4Na > C12E6 > C12E5 > C12E3. This classification is different from that obtained when the phase inversion is caused by a change in temperature (PIT-slope method) because the addition of NaCl in significant amounts (3 to 10 wt%) partially screens the ionic heads and diminishes their apparent hydrophilicities. A simple model, valid for all types of nonionic surfactants, is developed on the basis of the HLDN equation (Normalized Hydrophilic-Lipophilic Deviation) to express the SPI-slope as a function of the hydrophilic/lipophilic ratio (PACN2) and the salinity coefficient (δ2) of S2. All studied surfactants are positioned on a 2D map according to the values of their SPI-slope and their PIT-slope to graphically highlight their hydrophilic/lipophilic ratio and their salt-sensitivity. Finally, a linear model connecting the PIT-slope and the SPI-slope is derived for nonionics, emphasizing that the thermal partitioning of C10E4 towards n-octane is much greater in the PIT-slope than in the SPI-slope experiments.
Ontiveros, J. F.; Hong, B.; Aramaki, K.; Pierlot, C.; Aubry, J.-M.; Nardello-Rataj, V.
J Surfactants Deterg 2021, 24 (3), 401–410.
A series of symmetrical dialkyl methanesulfonate amphiphiles [DiCnCHSO3]mM (n = 6, 7, 8) with different counter cations (Mm+ = H+, Li+, Na+, K+, Cu2+, Zn2+, Mg2+, Ca2+, Sc3+) were synthesized in five steps. Their solubility and critical micelle concentration (CMC) in water were determined highlighting a huge effect of the chain length and the nature of the cation. The hydrophilic–lipophilic balance of the surfactants were assessed with the phase inversion temperature (PIT)-slope method based on the deviation from the PIT of the reference C10E4/n-octane/0.01 M NaCl(aq) emulsion through addition of increasing amounts of the dialkyl methanesulfonates. The hydrophilicity of the surfactants was thus ranked in terms of dPIT/dxsulfonate. A “cation” scan with the [DiC6CHSO3]mM/benzene/water systems at fw = 0.5 was finally performed confirming the hydrophilicity ranking obtained with the PIT-slope method. It revealed that the [DiC6CHSO3]2Mg behaves as a “Balanced Surfactant” able to form spontaneously a three-phase microemulsion system (Winsor III) just in the presence of water and oil, in the same way as the catalytic surfactant dimethyldioctylammonium molybdate, which has the same PIT-slope.
Delforce, L.; Hofmann, E.; Nardello-Rataj, V.; Aubry, J.-M.
Colloids Surf. A Physicochem. Eng. Asp. 2021, 628, 127333.
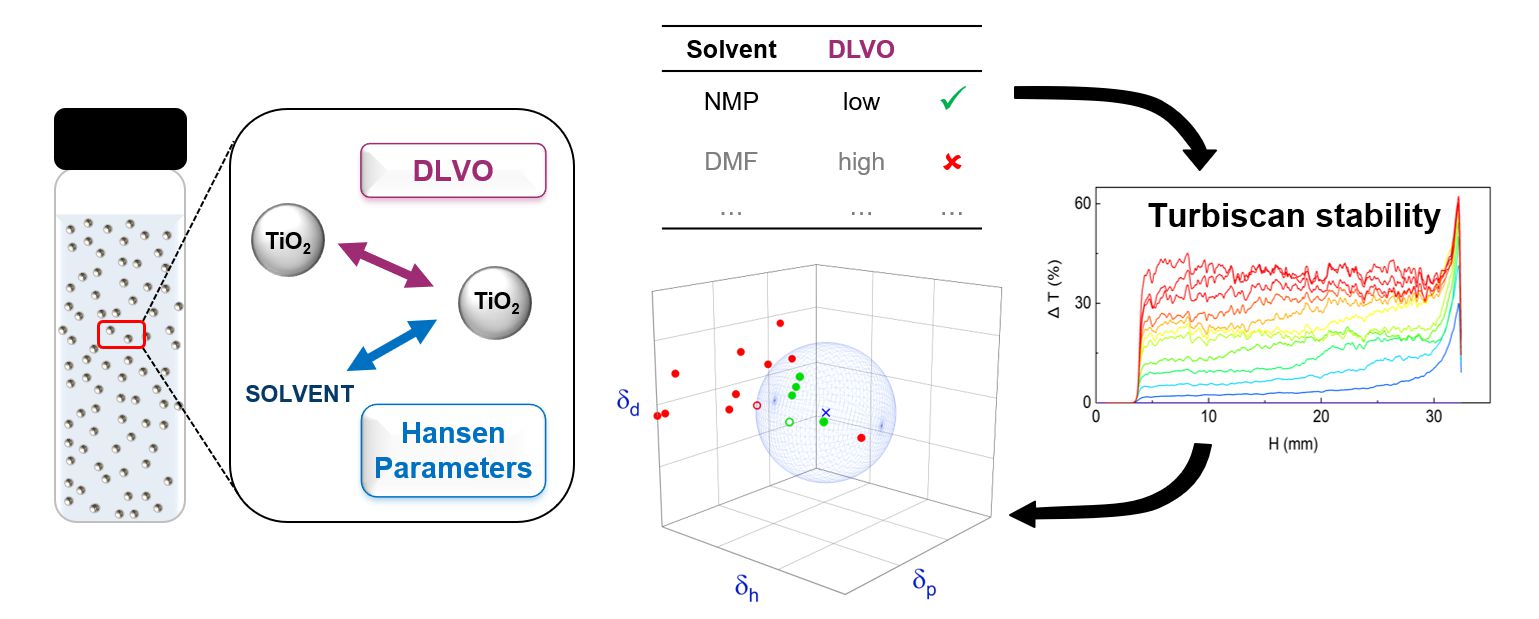 Hansen Solubility Parameters (HSP) have been shown to be an effective approach for rationalizing and predicting the stability of titanium dioxide nanoparticles (TiO2 NPs) dispersions. However, interparticle electrostatic interactions, not considered in Hansen’s approach but taken into account in the DLVO theory, are expected to play a significant role in organic solvents having a notable dielectric constant. Zeta potential ζ of TiO2 P25 NPs was measured in both aqueous and organic media to identify DLVO-stabilized dispersions from those stabilized by more specific NP-solvent interaction. Stability was quantified using a Turbiscan optical device which provides Stokes diameters and Relative Turbiscan Stability Index (RTSI). When the zeta potential of NPs and the dielectric constant of the solvent are both high, the dispersion benefits from additional stabilization while when the electrostatic repulsion is negligible, only the solvents within a Hansen dispersion sphere give stable dispersions. The two interpretations are therefore complementary to describe the behavior of TiO2 dispersions in organic solvents.
Hansen Solubility Parameters (HSP) have been shown to be an effective approach for rationalizing and predicting the stability of titanium dioxide nanoparticles (TiO2 NPs) dispersions. However, interparticle electrostatic interactions, not considered in Hansen’s approach but taken into account in the DLVO theory, are expected to play a significant role in organic solvents having a notable dielectric constant. Zeta potential ζ of TiO2 P25 NPs was measured in both aqueous and organic media to identify DLVO-stabilized dispersions from those stabilized by more specific NP-solvent interaction. Stability was quantified using a Turbiscan optical device which provides Stokes diameters and Relative Turbiscan Stability Index (RTSI). When the zeta potential of NPs and the dielectric constant of the solvent are both high, the dispersion benefits from additional stabilization while when the electrostatic repulsion is negligible, only the solvents within a Hansen dispersion sphere give stable dispersions. The two interpretations are therefore complementary to describe the behavior of TiO2 dispersions in organic solvents.
Ontiveros, J.F.; Pierlot, C.; Catté, M.; Molinier, V.; Salager ,J-L.; Aubry, J-M.
J. Colloid Interface Science. 2015, 48, 222-230
The Phase Inversion Temperature of a reference C10E4/n-Octane/Water system exhibits a quasi-linear variation versus the mole fraction of a second surfactant S2 added in the mixture. This variation was recently proposed as a classification tool to quantify the Hydrophilic–Lipophilic Balance (HLB) of commercial surfactants. The feasibility of the so-called PIT-slope method for a wide range of well-defined non-ionic and ionic surfactants is investigated. The comparison of various surfactants having the same dodecyl chain tail allows to rank the polar head hydrophilicity as: SO3Na ⩾ SO4Na ⩾ NMe3Br > E2SO3Na ≈ CO2Na ⩾ E1SO3Na ⩾ PhSO3Na > IsosorbideexoSO4Na ≫ IsosorbideendoSO4Na ≫ E8 ⩾ NMe2O > E7 > E6 ⩾ Glucosyl > E5 ⩾ Diglyceryl ⩾ E4 > E3 > E2 ≈ Isosorbideexo > Glyceryl > Isosorbideendo. The influence on the surfactant HLB of other structural parameters, i.e. hydrophobic chain length, unsaturation, replacement of Na+ by K+ counterion, and isomerism is also investigated. Finally, the method is successfully used to predict the optimal formulation of a new bio-based surfactant, 1-O-dodecyldiglycerol, when performing an oil scan at 25 °C.

Lukowicz, T.; Benazzouz, A.; Nardello-Rataj, V.; Aubry, J-M.
Langmuir 2015, 31, 41, 11220–11226

The equivalent alkane carbon numbers (EACNs) of 20 polar hydrocarbon oils are determined by the fishtail method. These values supplemented by 43 already reported EACNs of other hydrocarbons are rationalized by using the COSMO-RS σ-moments as descriptors for a QSPR analysis. A reliable model, with only two meaningful physicochemical parameters, namely the surface area (M0X) and the overall polarity (M2X) of the oil X, is able to predict the EACN values of a large variety of oils including (cyclo)alkanes, (cyclo)alkenes, terpenes, aromatics, alkynes, and chloroalkanes and to rationalize structural effects on EACNs. Furthermore, the dependence of the EACN of homologous oils on the chain length provides some molecular insight into how the different oils penetrate into the interfacial film of surfactants.
Applications et propriétés des matières premières biosourcées
Moity, L.; Molinier, V.; Benazzouz, A.; Joossen, B.; Gerbaud, V.; Aubry, J-M.
Green Chem. 2016, 18, 3239-3249
Potentially effective glycerol-based solvents for nitrocellulose have been designed using a top-down in silico procedure that combines Computer Assisted Organic Synthesis (CAOS) and Molecular Design (CAMD). Starting from a bio-based building block – glycerol – a large number of synthetically feasible chemical structures have been designed using the GRASS (GeneratoR of Agro-based Sustainable Solvents) program. GRASS applies well-selected industrial chemical transformations to glycerol together with a limited number of relevant co-reactants. Then, the most promising structures are considered as lead compounds for further modification in silico thanks to the IBSS (InBioSynSolv) program, which generates derivatives with alkyl, cycloalkyl, alkene, cycloalkene or phenyl substituents. Finally, IBSS ranks all the candidates according to the value of their overall performance function to best fit the predefined specifications, i.e. (i) high solubilisation of nitrocellulose, (ii) slow evaporation and non-flammability (iii) low toxicity and environmental impact. This general strategy enables the highlighting of the most relevant solvent candidate derived from any building block for a given application. To validate the approach, 15 commercially available solvents derived from glycerol were confronted with nitrocellulose and led to highlight diacetin as an effective and safe solvent.

Catalyse et oxydation
Mouret, A.; Leclercq, L.; Mühlbauer, A.; Nardello-Rataj, V.
Green Chem. 2014, 16, 269. DOI: 10.1039/C3GC42032A
Eighteen eco-friendly solvents were examined to carry out the epoxidation of olefins with the amphiphilic catalytic dodecyltrimethylammonium
polyoxometalate nanoparticles [C12]3[PW12O40] in comparison with [H]3[PW12O40] and [Na]3[PW12O40]. Surprisingly, the screening of solvents with cyclooctene has revealed that the [C12]3[PW12O40] catalyst is much more active with initial turn-over frequencies, TOF0, increasing up to a factor of 10. Moreover, the reaction occurs at competitive rates in four relevant solvents, i.e. cyclopentyl methyl ether, 2-methyl tetrahydrofuran, methyl acetate and glycerol triacetate, for which TOF0 values are higher than 260 h−1. The recyclability of the systems is demonstrated and the scope of
substrates has been successfully extended to cyclohexene, 1-octene, limonene, 3-carene, α-pinene, β-pinene and neryl acetate with good epoxide selectivity. The catalytic performances in the “green” solvent are assigned to the formation of stable [C12]3[PW12O40] nanoparticle dispersions which have been characterized by transmission electron microscopy and dynamic and multiple light scattering experiments. Finally, the Kamlet–Taft parameters were measured in order to correlate the physicochemical properties of the solvents and the catalytic activity.
Pera-Titus, M.; Leclercq, L.; Clacens, JM.; De Campo, F.; Nardello-Rataj, V.
Angew. Chem. Int. Ed. 2015, 28, 316
Pickering emulsions are surfactant-free dispersions of two immiscible fluids that are kinetically stabilized by colloidal particles. For ecological reasons, these systems have undergone a resurgence of interest to mitigate the use of synthetic surfactants and solvents. Moreover, the use of colloidal particles as stabilizers provides emulsions with original properties compared to surfactant-stabilized emulsions, microemulsions, and micellar systems. Despite these specific advantages, the application of Pickering emulsions to catalysis has been rarely explored. This Minireview describes very recent examples of hybrid and composite amphiphilic materials for the design of interfacial catalysts in Pickering emulsions with special emphasis on their assets and challenges for industrially relevant biphasic reactions in fine chemistry, biofuel upgrading, and depollution.
